Ammonia Nitrogen In Water

Unlike other forms of nitrogen which can cause nutrient over enrichment of a water body at elevated concentrations and indirect effects on aquatic life ammonia causes direct toxic effects on aquatic life.
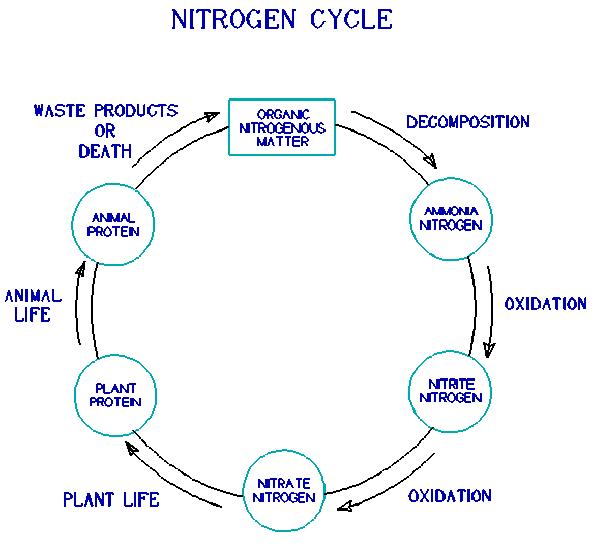
Ammonia nitrogen in water. Ammonia can also be found in most water sources as a byproduct of organic decomposition processes especially the decomposition of proteins which contain nitrogen. Alternatively factors from table 1 may be multiplied by total ammonia nitrogen concentration to give the ammonia concentration as the amount of nitrogen in ammonia nh 3 n. Together these two forms of ammonia are called tan which means total ammonia nitrogen nh3 is the principal form of toxic ammonia. Ammonia is a colorless pungent gaseous compound of hydrogen and nitrogen that is highly soluble in water.
It also may find its way to ground and surface waters through discharge of industrial process wastes containing ammonia and fertilizers. It combines n 2 from air and hydrogen gas using a catalyst temperatures above 400 c and. Ammonia is a colorless pungent gaseous compound of hydrogen and nitrogen that is highly soluble in water. Analytical method ammonia and ammonium cation at concentrations between 0 025 and 3 mg litre can be determined by the indophenol reaction 1 2 5 6.
Water than ammonia and is involved in the biological processes of nitrogen fixation mineralization and nitrification 2. It is a biologically active compound found in most waters as a normal biological degradation. The ammonia concentration must be calculated from the total ammonia nitrogen concentration by aid of the equilibrium constant for one of the reactions shown above ph and water temperature. Ammonia is one of several forms of nitrogen that exist in aquatic environments.
Nh 3 this is called unionized ammonia nh 4 this is called ionized ammonia. Ammonia exists in two forms in the water. It is a biologically active compound found in most waters as a normal biological degradation product of nitrogenous organic matter protein. The haber bosch process is used to make ammonia the raw material for nitrogen based fertilizers.
It can also be used as a measure of the health of water in natural bodies such as rivers or lakes or in man made water reservoirs.


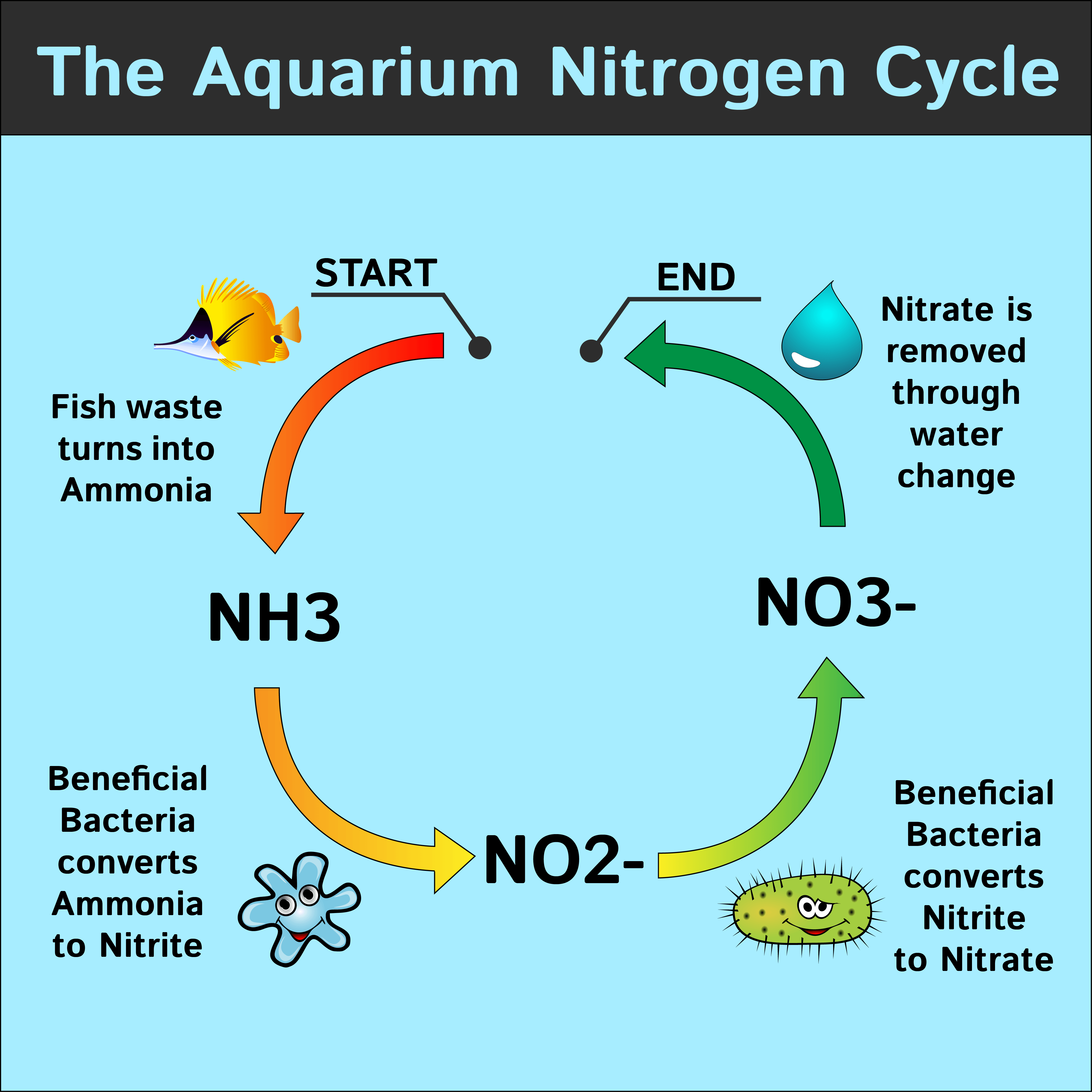

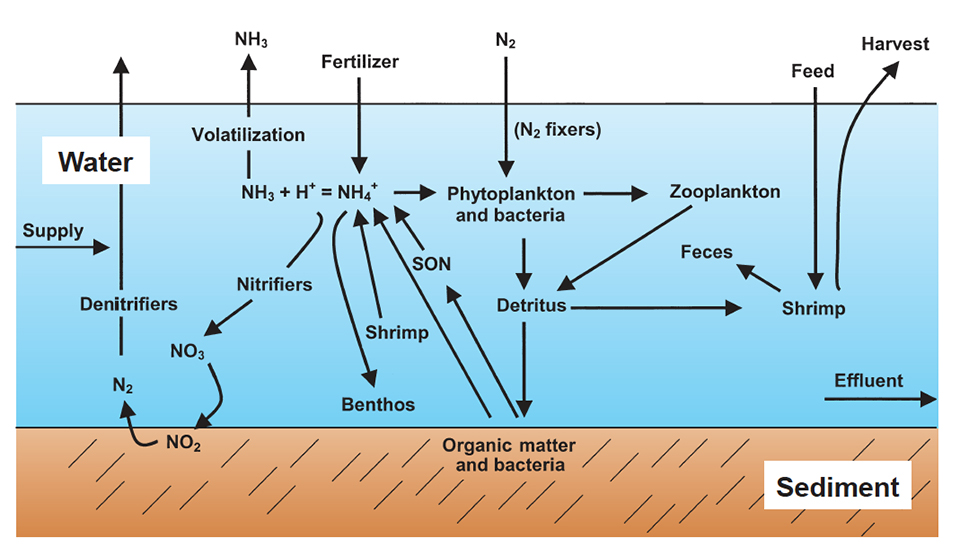



:max_bytes(150000):strip_icc()/NitrogenCycle-5667357c3df78ce161d649cf.jpg)