Impressed Current Cathodic Protection On Ships

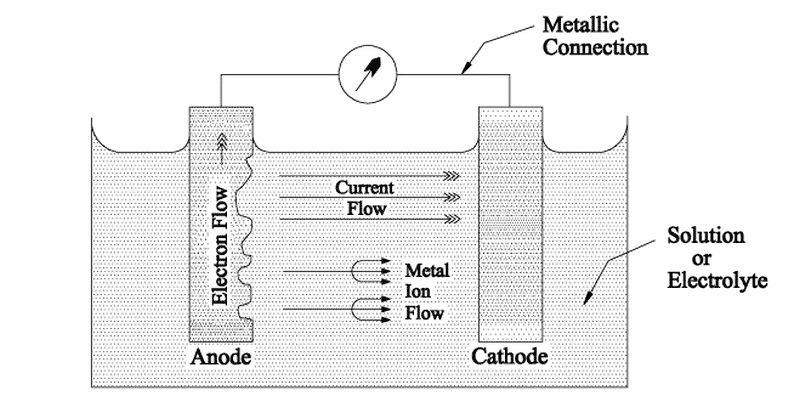
As you know when two metals having different electrode potentials come into contact in an electrolyte then one will become anode and other act as cathode.
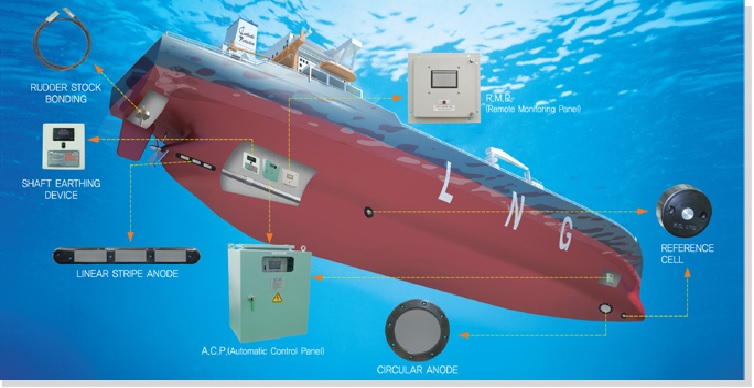
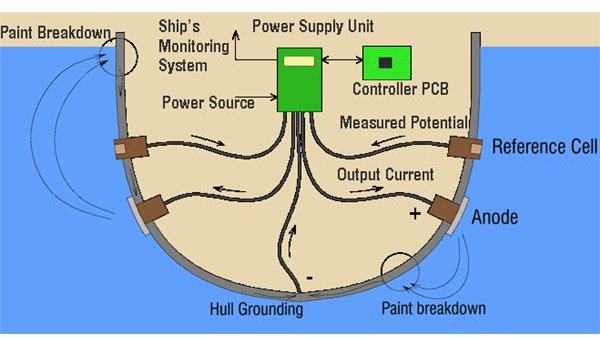
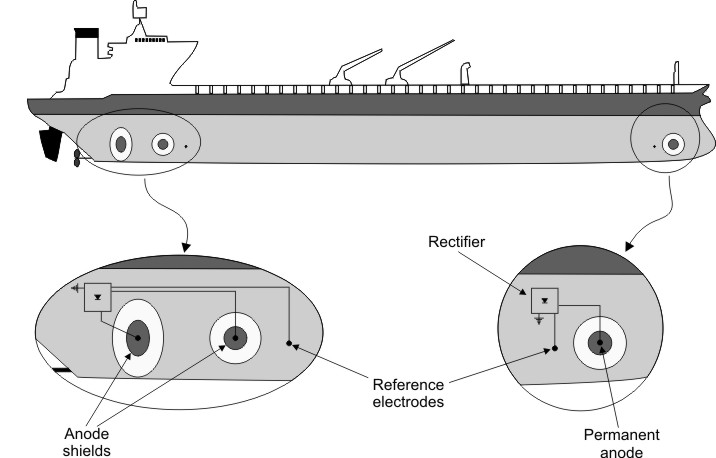
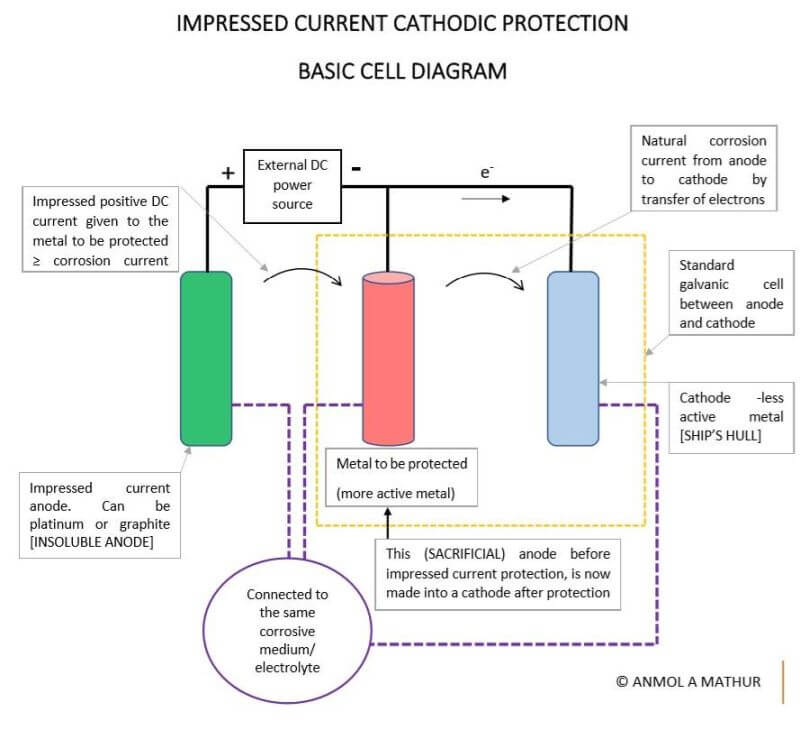
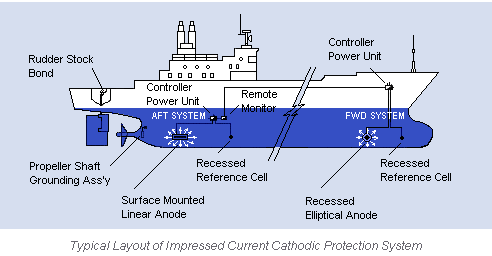
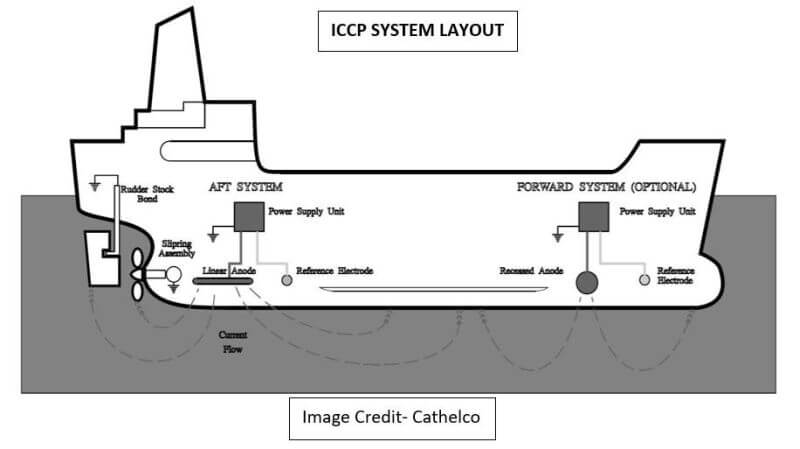
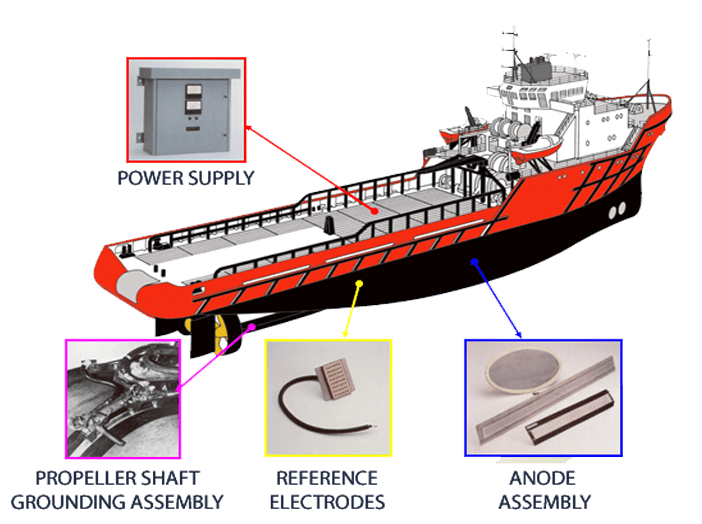
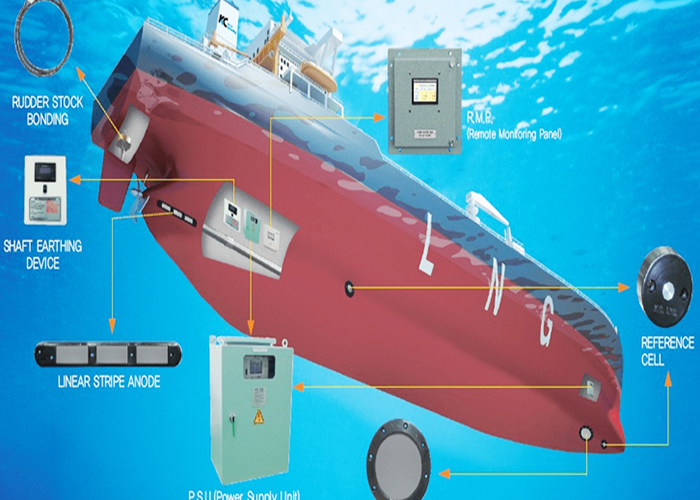
Impressed current cathodic protection on ships. It would be 100 years after davy s experiment before cathodic protection was used widely on oil pipelines in the united states 5 cathodic protection was applied to steel gas pipelines beginning in 1928 6 and more. As we now know the problem and as how we can solve it. Impressed current cathodic protection iccp systems consist of one or more reference electrodes and several iccp anodes which are all connected to a power unit. This article will describe how these two systems are used and what are their advantages and disadvantges.
Impressed current cathodic protection iccp is a corrosion protection system consisting of sacrificial anodes connected to an external power source. Impressed current cathodic protection on ships iccp corrosion by sea water is an electrochemical process in which the ship hull acts as electrode and sea water as electrolyte. Impressed current cathodic protection on ship. So now negative ions formed at cathode will combine with positive ions at the anode and gets rusted.
Cathodic protection does not appear to deter molluscular growth on the ships hull so a top coat of anti foul poisonous paint is still necessary. Sacrificialanode iccp hullcorrosion ship s hull corrosion is one of the biggest worries for seafarers as the vessel works in a highly corrosive environmen. Thomas edison experimented with impressed current cathodic protection on ships in 1890 but was unsuccessful due to the lack of a suitable current source and anode materials. The total impressed current for a hull in good condition may be as low as 20 a.
The chief among them which are widely used are sacrificial anodes and impressed current cathodic protection system. The previous article dealt with the process of corrosion and the different reactions involved in it. For more than 25 years sea going vessels of every type and size oil tankers lng carriers cruise ships pleasure craft workboats semi submersibles and more have benefited from the 24 hour protection provided by impressed current cathodic protection systems against the costly corrosive effects of electrolysis. This article will deal with the different ways to fight corrosion on ship.
The external power source often a dc power supply provides the current necessary to drive the electrochemical reaction required for cathodic protection to occur.