Impressed Current Cathodic Protection
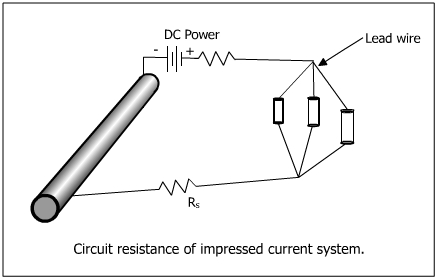
Impressed current cathodic protection systems have the benefit of using an external power supply to drive current.
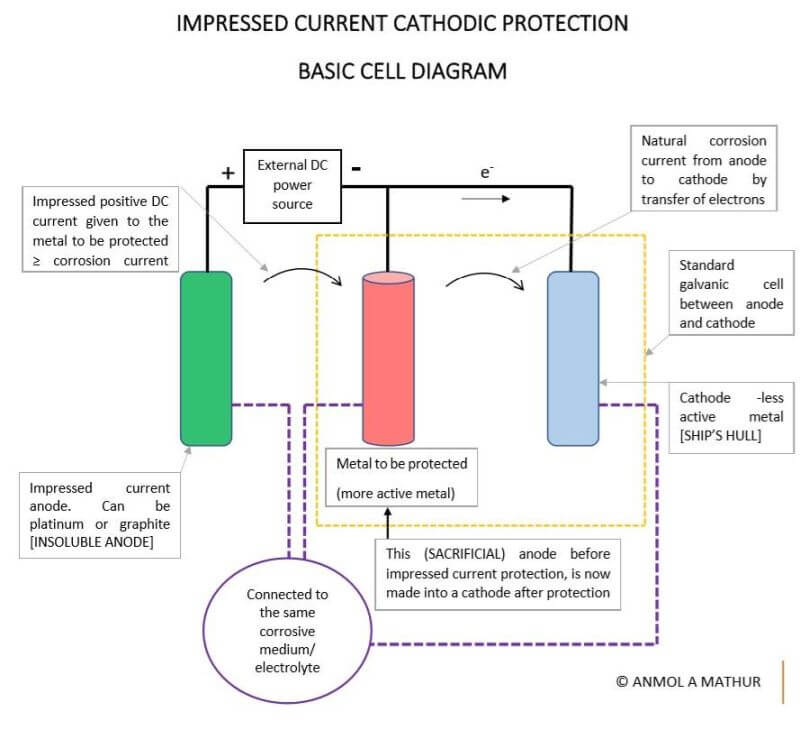
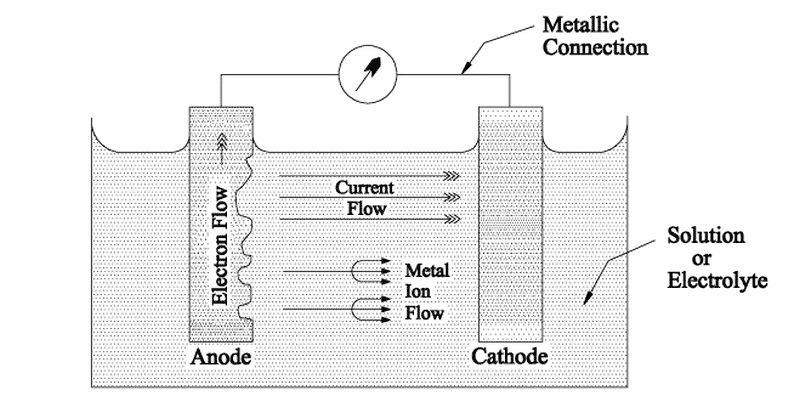
Impressed current cathodic protection. Impressed current cathodic protection systems are the technologically advanced and long term solution to corrosion problems and is regarded as a superior alternative to sacrificial anode systems. Impressed current cathodic protection iccp is a corrosion protection system consisting of sacrificial anodes connected to an external power source. Impressed current cathodic protection on ships iccp corrosion by sea water is an electrochemical process in which the ship hull acts as electrode and sea water as electrolyte. Impressed current is a type of cathodic protection utilizing electrochemical means to obtain protection against corrosion.
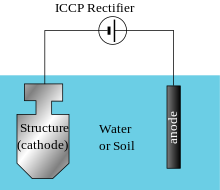
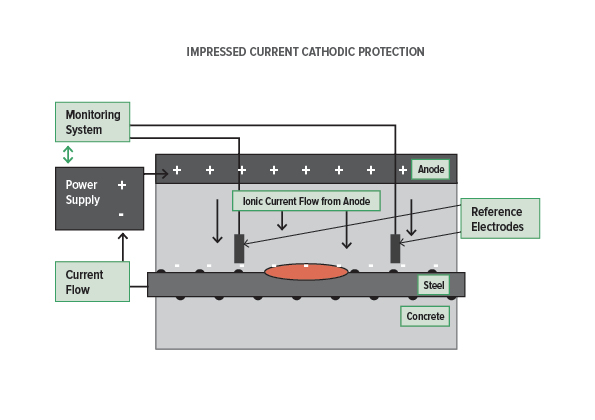
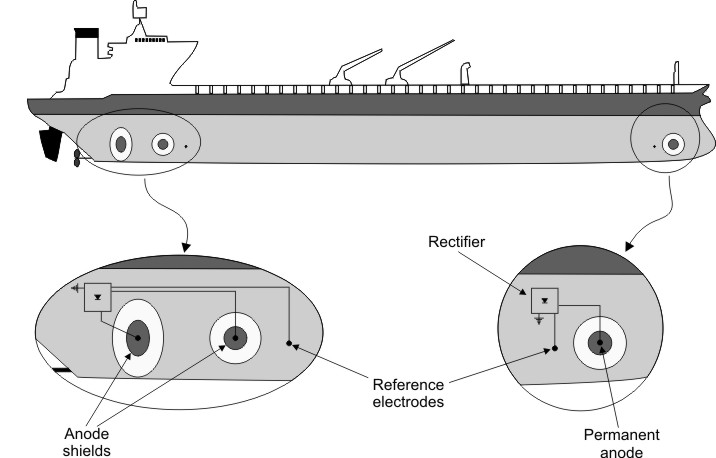
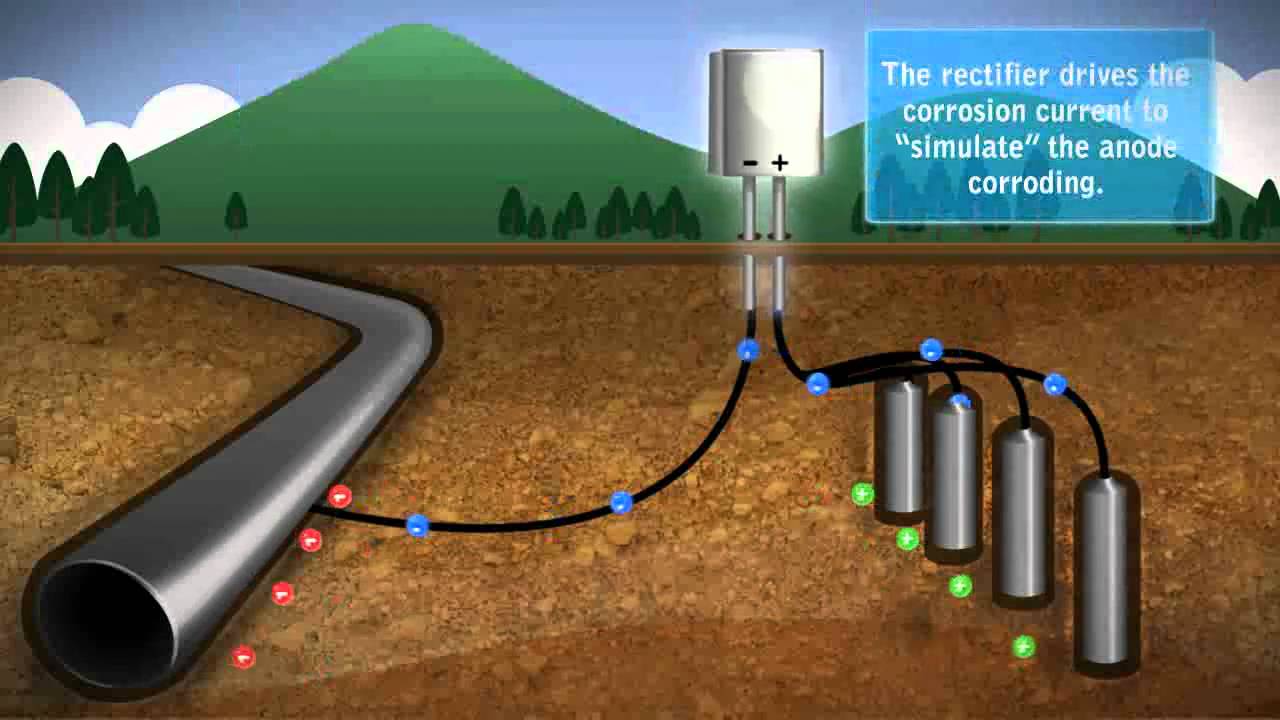
The reference cells measure the underwater electrical protection potential and based on this data the power unit regulates the required output to the anodes. Impressed current cathodic protection iccp systems consist of one or more reference electrodes and several iccp anodes which are all connected to a power unit. The external power source often a dc power supply provides the current necessary to drive the electrochemical reaction required for cathodic protection to occur. Impressed current cathodic protection iccp impressed current cathodic protection is applied by coupling the metal to be protected to the negative pole of a direct current dc source while the positive pole is coupled to an auxiliary anode since the driving voltage is provided by the dc source there is no need for the anode to be more active than the structure to be protected.
Impressed current cathodic protection systems are preferred by ship owners because they reduce fuel cost and maintenance. Our systems work by supplying a controlled amount of dc current to submerged surfaces using highly reliable mixed metal oxide anodes and zinc reference electrodes. As you know when two metals having different electrode potentials come into contact in an electrolyte then one will become anode and other act as cathode. This makes it possible to protect virtually any structure regardless of size or current requirements using long life anodes and enough appropriately sized power supplies.
Thomas edison experimented with impressed current cathodic protection on ships in 1890 but was unsuccessful due to the lack of a suitable current source and anode materials.